Abstract
Background:
Genetic and functional drivers of diffuse large B-cell lymphoma (DLBCL) have increasingly been used to prognosticate and occasionally even guide treatment for patients with advanced stage DLBCL. In advanced disease, the non-germinal center (non-GC) phenotype, double hit lymphomas (DHLs), and double expression lymphomas (DELs) have poorer outcomes. However, the incidence and impact of these factors on patient outcomes in the limited stage setting is not as well described. In particular, the prevalence of MYC and BCL2 overexpression has not been previously described in the literature for early stage DLBCL. The purpose of our analysis was to determine the incidence of these high-risk factors and analyze if these have an impact on outcomes in patients with stage I-II DLBCL.
Methods:
We performed a single site retrospective study of patients newly diagnosed with stage I-II DLBCL as defined by the Ann-Arbor staging system. All patients were treated at our institution, between 2011 and 2017. Patients with stage III-IV disease, concomitant follicular lymphoma, primary mediastinal lymphoma, or CNS lymphomas were excluded. The stage-modified IPI was used to categorize patients into different risk categories.
Cell of origin (COO) was determined by the Hans criteria. Double expression was defined as overexpression of both MYC (positive ≥50%) and BCL2 (positive ≥70%). Molecular rearrangements of MYC with either BCL2 or BCL6 were used to define DHLs. MYC overexpression, BCL2 overexpression, double (MYC and BCL2) expression, MYC rearrangement, and Ki67 were quantified. Whenever possible, original patient histologic tissue samples were examined if information was not available in the original pathology report.
The study examined the impact of these clinical and pathologic factors on progression-free survival (PFS). The impact of prognostic factors was analyzed with the Wilcoxon rank-sum for continuous variables and chi-square tests for categorical variables.
Results:
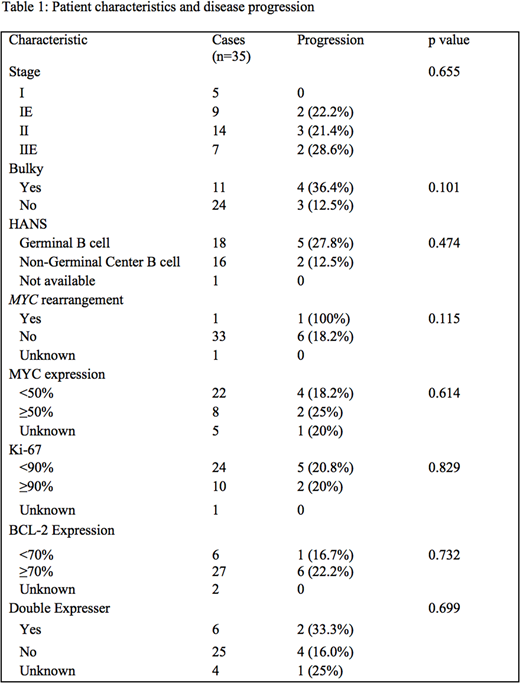
A total of 35 patients were identified with stage I-II DLBCL. The median age was 60 years old (range 25-86). Twelve patients (34%) were female. Patient characteristics are summarized in Table 1. Fifteen patients (43%) were treated with chemotherapy alone, while 20 patients (57%) were treated with a combination of chemotherapy and radiation.
There were 8 patients (23%) with MYC overexpression and 27 patients (77%) with BCL2 overexpression. Six patients (17%) had double expression. Of the 6 patients who were double expressers, 2 (33.3%) progressed after frontline therapy, while 4 of 25 non-double expressers progressed (16%), although this was not a statistically significant difference. The COO was the non-GC type in 16 patients (45.7%) by Hans criteria. There was 1 patient with MYC rearrangement, and this patient had progression at distant sites. We did not identify any DHLs. There was 1 patient with CNS relapse, but this patient did not have double expression or MYC rearrangement. There was no significant difference in PFS associated with any of our identified clinical and pathological factors.
Conclusion:
In this retrospective review, we identified the proportion of patients who have high risk genetic and pathologic risk factors. The prognostic impact of the COO in localized DLBCL has been described, and our data support the previously published studies showing no difference in localized DLBCL (Savage et al., Blood 2016 and Johnson et al., JCO 2012). The prognostic impact of DELs has been well described in advanced stage DLBCL; however, the prognostic impact of DELs in localized DLBCL is not well described. To our knowledge, this is the first study reporting the frequency of MYC and BCL2 overexpression in localized DLBCL. While it is difficult to compare rates across trials, the rate of patients with double expression at 17% is lower than the rate of approximately 30% described for advanced stage DLBCL (Johnson et al., JCO 2012 and Hu et al., Blood 2013). Additionally, there was not a significant difference in the risk of progression for DEL versus non-DEL, but this was limited by the small sample size.
Grover:Seattle Genetics: Consultancy. Dittus:Seattle Genetics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal